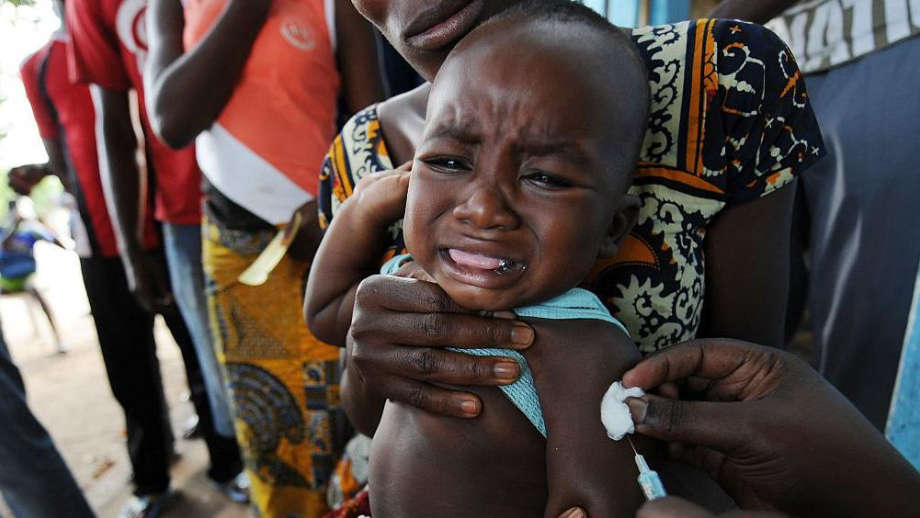
The World Health Organization has criticised a proposed U.S.-funded hepatitis B vaccine trial involving newborns in Guinea-Bissau, describing the study as “unethical” over concerns it could expose infants to preventable harm.
The now-suspended study, funded by the U.S. Centers for Disease Control and Prevention and led by Danish researchers, planned to enrol about 14,000 babies.
It sought to administer the hepatitis B vaccine to one group at birth while delaying the shot for another group until six weeks.
The WHO said the trial raised “significant concerns” over its scientific justification, ethical safeguards and compliance with international research standards.
It stressed that the hepatitis B birth-dose vaccine is a proven life-saving intervention used in more than 115 countries for over three decades.
“Giving a proven life-saving intervention to some newborns but not others expose them to potentially irreversible harm,” the agency said.
The WHO recommends vaccination within 24 hours of birth, noting that infection at birth is the leading cause of lifelong hepatitis B, with about 90 percent of infected newborns becoming chronic carriers.
Guinea-Bissau suspended the project last month following public backlash and criticism from health experts, who questioned the choice of the West African country for the study.
The U.S. health department, led by Robert F. Kennedy Jr., had backed the research to examine the vaccine’s broader health effects.
Former Guinea-Bissau health minister Magda Robalo opposed the plan, saying, “Guinea-Bissauans are not guinea pigs.”
Hepatitis B remains widespread in Guinea-Bissau, with more than 12 percent of adults living with chronic infection.
The country currently administers the vaccine at six weeks but plans to introduce the birth dose nationwide by 2028, with WHO support.
The global health body reiterated that delayed-treatment trials are only justified where no proven intervention exists, stressing that the hepatitis B birth-dose vaccine does not meet that condition.